Abstract
Introduction: Host genetic variations have an essential role in the mutational landscape of Philadelphia-negative myeloproliferative neoplasm (MPNs), with JAK2 46/1 and TERT rs2736100 polymorphisms predisposing to disease onset (Tapper W et al, Nat Commun. 2015). However, the contribution of inherited factors in disease phenotype and evolution is poorly characterized. We recently demonstrated that the -2518 A/G SNP of the Monocyte Chemoattractant Protein-1 (MCP-1, rs1024611) is an inherited host genetic factor associated with secondary myelofibrosis (sMF) and a biomarker of disease severity in MF, correlating with adverse hematologic characteristics at the time of diagnosis, such as higher IPSS risk category (Masselli E et al, Leukemia 2018). Here we aimed to: i) provide a correlation between MF patients' genetic readout, chemokine expression/production and hematopoietic progenitor differentiation, ii) correlate MCP-1 levels with disease subtype and severity.
Methods: 16 MF patients were genotyped for MCP-1 SNP by TaqMan Predesigned SNP Genotyping Assays and added to our previously reported dataset (Masselli E et al, Leukemia 2018), reaching 81 MF (20 prePMF, 36 overtPMF and 25 sMF). Samples from 15 therapy-naïve MF were dedicated to in vitro-experiments. Eleven healthy subjects and 4 apheresis bags were utilized as controls (CTRL). MF were stratified according to their rs1024611 genotype in A/A (wild types), A/G and G/G (polymorphics). Peripheral blood mononuclear cells (MNCs) were isolated by Ficoll-Hypaque gradient, in part pelleted (resting, T0) and in part seeded in RPMI-1640 medium and activated with 1.1 ng/ml of IL-1β for 20h (T1). CD34+-cells were purified from MF peripheral blood and from apheresis by immunomagnetic selection and differentiated toward the MK lineage as described in Masselli E et al, Leukemia 2015, up to 14 days. MK differentiation was assessed by CD41-flow cytometric expression. MCP-1 levels were evaluated by real-time PCR and western blot.
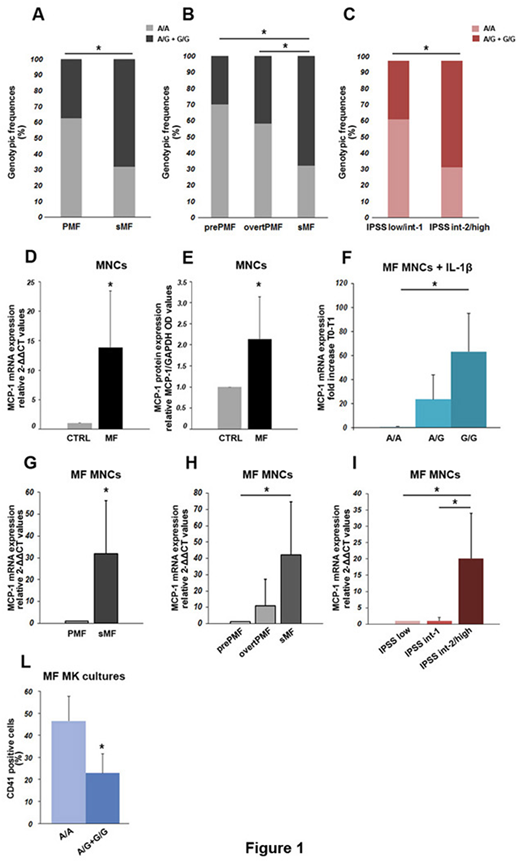
Results: We confirmed, in a cohort of 81 MF patients, that polymorphic subjects were significantly more frequent in sMF vs. PMF (17/25, 68% vs. 21/56, 37.5%, respectively, P=.011, Fig.1A). Of note, sMF was significantly more frequent in A/G and G/G individuals than either prePMF (6/20, 30%, P=.011) or overtPMF (15/36, 41.7%, P=.034, Fig.1B). In overall MF patients, a higher frequency of allele-G carriers was also confirmed in IPSS int-2/high vs. low/int-1 risk group (17/25, 68% vs. 19/51, 37.3%, respectively, P=.011, Fig.1C - all comparisons made by Χ2 test).
We proved that MF MNCs significantly over-expressed MCP-1 mRNA and protein as compared to CTRL MNCs at basal state (P=.04 and P=.006 by t-test, respectively, Fig.1D-E), consistently with reports from Tefferi et al. on serum ELISA (Tefferi A et al, JCO 2011). Of note, basal MCP-1 mRNA levels in MF correlated with genotypes, with polymorphic patients displaying significantly higher transcript levels vs. wild types (~20-times higher 2-ΔΔCT, P=.018 by t-test). When MF MNCs were stimulated ex-vivo with IL-1β, a dose-dependent effect of the SNP on MCP-1 expression was observed, with A/G patients displaying >20-times higher and G/G patients showing >60-times-higher fold-increase in MCP-1 expression as compared to wild types (P<.05 G/G vs. A/A by ANOVA and Dunnett test, Fig.1F). sMF patients, that we proved to be enriched in polymorphic genotypes as compared to overall PMF, prePMF and overtPMF patients, significantly over-expressed MCP-1 as compared to PMF (P<.0001 by t-test, Fig. 1G) and prePMF (P<.05 by Anova and Dunnett test, Fig. 1H). Similarly, IPSS intermediate-2/high risk MF patients, also enriched in the G allele, showed significantly higher MCP-1 mRNA levels vs. both IPSS low and intermediate-1 (P<.05 by ANOVA and Dunnett test, Fig. 1I). Finally, we demonstrated that polymorphic CD34+-cells displayed impaired megakaryocytic differentiation, indicated by a significantly lower number of CD41+-cells obtained in culture as compared to A/A (P=.04 by t-test, Fig.1L).
Conclusions: Our data proved a dose-dependent effect of the rs1024611 SNP on MCP-1 production in MF and establish a correlation between patients' genetic readout and chemokine levels. These results suggest that MF patients harboring the G allele - and thus producing more MCP-1 - are genetically prone to a higher inflammatory burden, to display a more severe disease and abnormal MK differentiation.
Aversa:Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria; Astellas: Honoraria; Basilea: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal